Medical examinations in guinea pigs
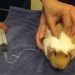
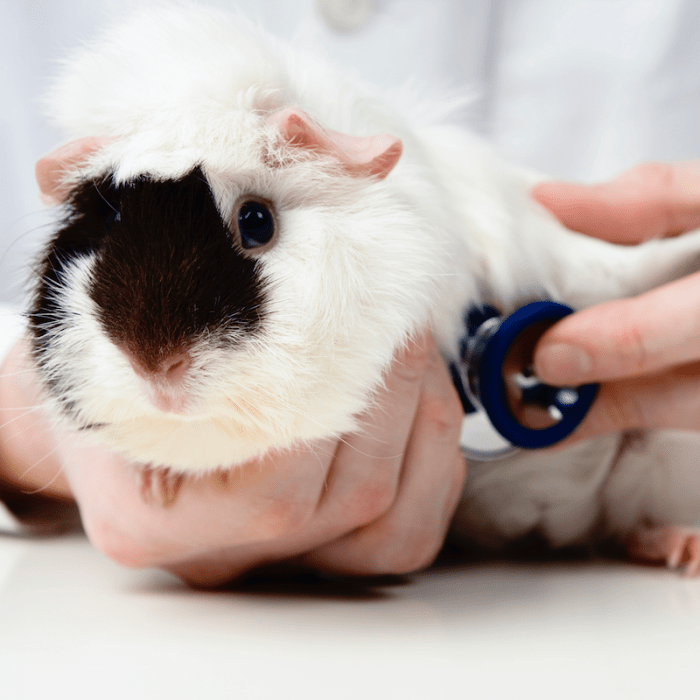
Guinea pigs are pronounced peaceful animals, in relation to which there is no need to use coercion. However, if they need, for example, medical attention, they get scared, trying to escape. In this case, it is recommended to keep animals. Although sometimes it is enough to take the wool at the back of the head, which limits freedom of movement.
Guinea pigs are pronounced peaceful animals, in relation to which there is no need to use coercion. However, if they need, for example, medical attention, they get scared, trying to escape. In this case, it is recommended to keep animals. Although sometimes it is enough to take the wool at the back of the head, which limits freedom of movement.
Contents
Taking blood from guinea pigs
With some skill, guinea pigs can take blood from the vena cephalica. To do this, stop the flow of blood over the elbow with a rubber bandage and stretch the limb of the animal. If necessary, you can cut the hair with scissors. After disinfection with a swab dipped in alcohol, carefully insert the N16 needle. Blood is removed directly from the cone of the needle. If only one drop is needed for a smear, then after a vein puncture, it can be removed directly from the skin.
Another possibility of taking blood is a puncture of the venous plexus of the orbit of the eye. After anesthetizing the eye with a few drops of Ophtocain, turn the eyeball outward with the index finger. Then carefully introduce a hematocrit microtubule under the eyeball to the venous plexus of the orbit. When the tube reaches behind the orbital plexus, the vessels rupture easily and fill the capillary tube with blood. After taking the blood, it is enough to press lightly for 1-2 minutes on the closed eyelid to stop the bleeding. This method requires the skill of the veterinarian, as well as the calm state of the patient.
With some skill, guinea pigs can take blood from the vena cephalica. To do this, stop the flow of blood over the elbow with a rubber bandage and stretch the limb of the animal. If necessary, you can cut the hair with scissors. After disinfection with a swab dipped in alcohol, carefully insert the N16 needle. Blood is removed directly from the cone of the needle. If only one drop is needed for a smear, then after a vein puncture, it can be removed directly from the skin.
Another possibility of taking blood is a puncture of the venous plexus of the orbit of the eye. After anesthetizing the eye with a few drops of Ophtocain, turn the eyeball outward with the index finger. Then carefully introduce a hematocrit microtubule under the eyeball to the venous plexus of the orbit. When the tube reaches behind the orbital plexus, the vessels rupture easily and fill the capillary tube with blood. After taking the blood, it is enough to press lightly for 1-2 minutes on the closed eyelid to stop the bleeding. This method requires the skill of the veterinarian, as well as the calm state of the patient.
Urinalysis in guinea pigs
When examining the bladder of a guinea pig, it is gently squeezed out. However, animals excrete urine if they are placed on bedding covered with a crumpled plastic bag. As a rule, within an hour a sufficient amount is collected for examination.
It is not recommended to insert a catheter in males, as it is easy to damage the urethra. Urine in guinea pigs is alkaline and contains crystals of calcium carbonate and triple phosphate. The precipitate can be obtained in a hematocrit microcentrifuge.
When examining the bladder of a guinea pig, it is gently squeezed out. However, animals excrete urine if they are placed on bedding covered with a crumpled plastic bag. As a rule, within an hour a sufficient amount is collected for examination.
It is not recommended to insert a catheter in males, as it is easy to damage the urethra. Urine in guinea pigs is alkaline and contains crystals of calcium carbonate and triple phosphate. The precipitate can be obtained in a hematocrit microcentrifuge.
Litter examination in guinea pigs
A thorough examination of the litter is necessary when a new guinea pig is introduced into the house or in large groups of animals with frequent fluctuations. When keeping a single animal, examinations are necessary only in rare cases.
Endoparasites play only a minor role in domestic guinea pigs. To ascertain the presence of nematodes, a saturated solution of sodium chloride (specific gravity 1,2) is used. In a 100 ml plastic cup, mix well 2 g of litter and a little saturated sodium chloride solution. After that, the glass is filled to the brim with a solution of table salt, the contents are thoroughly stirred so that the particles of the litter are evenly distributed in the solution.
After 5 minutes, carefully place a coverslip on the surface of the solution. The floating testicles of worms will settle on it. After approximately one hour, the coverslip can be carefully removed from the solution using tweezers. The testicles are clearly visible under a microscope at a magnification of 10-40 times. During parasitological examination, 100 g of litter is stirred in tap water using the sedimentation technology in a 5 ml plastic cup in tap water so that a homogeneous suspension is obtained, which is filtered through a colander.
A few drops of dishwashing detergent are added to the filtrate, left for an hour, after which the top layer of liquid is poured off and refilled with water and detergent. After another hour, the water is drained again, and the sludge is well mixed with a glass rod. A few drops of sludge are placed on a glass slide with a drop of a 10% solution of methylene blue dye. The preparation is examined under a microscope at XNUMXx magnification without a cover slip. Methylene blue turns dirt particles and plants blue-black, and testicles yellow-brown.
A thorough examination of the litter is necessary when a new guinea pig is introduced into the house or in large groups of animals with frequent fluctuations. When keeping a single animal, examinations are necessary only in rare cases.
Endoparasites play only a minor role in domestic guinea pigs. To ascertain the presence of nematodes, a saturated solution of sodium chloride (specific gravity 1,2) is used. In a 100 ml plastic cup, mix well 2 g of litter and a little saturated sodium chloride solution. After that, the glass is filled to the brim with a solution of table salt, the contents are thoroughly stirred so that the particles of the litter are evenly distributed in the solution.
After 5 minutes, carefully place a coverslip on the surface of the solution. The floating testicles of worms will settle on it. After approximately one hour, the coverslip can be carefully removed from the solution using tweezers. The testicles are clearly visible under a microscope at a magnification of 10-40 times. During parasitological examination, 100 g of litter is stirred in tap water using the sedimentation technology in a 5 ml plastic cup in tap water so that a homogeneous suspension is obtained, which is filtered through a colander.
A few drops of dishwashing detergent are added to the filtrate, left for an hour, after which the top layer of liquid is poured off and refilled with water and detergent. After another hour, the water is drained again, and the sludge is well mixed with a glass rod. A few drops of sludge are placed on a glass slide with a drop of a 10% solution of methylene blue dye. The preparation is examined under a microscope at XNUMXx magnification without a cover slip. Methylene blue turns dirt particles and plants blue-black, and testicles yellow-brown.
Skin and coat tests in guinea pigs
Guinea pigs are often affected by mites, the presence of which is easy to identify. To do this, scrape a small surface of the skin with a scalpel until blood comes out. The resulting skin particles are placed on a glass slide, mixed with a 10% solution of caustic potassium and examined under a microscope at tenfold magnification two hours later. Another possibility for diagnosing ticks is the black paper test, which, however, is only necessary for severe lesions.
The patient is euthanized and placed on black paper. After a while, the mites move from the skin into the coat, where they can be easily seen with a strong magnifying glass or microscope. Sometimes they can be found on the blackest paper. Lice and lice are visible to the naked eye. However, practitioners are not recommended to use this method.
Another common problem is fungal diseases. The skin and coat samples taken must be sent to a mycological laboratory for diagnosis.
Guinea pigs are often affected by mites, the presence of which is easy to identify. To do this, scrape a small surface of the skin with a scalpel until blood comes out. The resulting skin particles are placed on a glass slide, mixed with a 10% solution of caustic potassium and examined under a microscope at tenfold magnification two hours later. Another possibility for diagnosing ticks is the black paper test, which, however, is only necessary for severe lesions.
The patient is euthanized and placed on black paper. After a while, the mites move from the skin into the coat, where they can be easily seen with a strong magnifying glass or microscope. Sometimes they can be found on the blackest paper. Lice and lice are visible to the naked eye. However, practitioners are not recommended to use this method.
Another common problem is fungal diseases. The skin and coat samples taken must be sent to a mycological laboratory for diagnosis.
X-ray examination of guinea pigs
The length and strength of the exposure for x-ray examination of guinea pigs depends on the cassette used and on the type of exposure and development. Good results can be achieved using exposure, which is used in x-ray examination of small cats.
The length and strength of the exposure for x-ray examination of guinea pigs depends on the cassette used and on the type of exposure and development. Good results can be achieved using exposure, which is used in x-ray examination of small cats.